A Crosstalk Between Pineal and Major Extra-Pineal Sources of Melatonin and its Role in Ovarian Growth and Maturation in Fish
DOI:
https://doi.org/10.18311/jer/2023/33014Keywords:
Extra-Pineal Sources, Fish Oocyte Growth and Maturation, Fish Reproduction, Melatonin, Pineal OrganAbstract
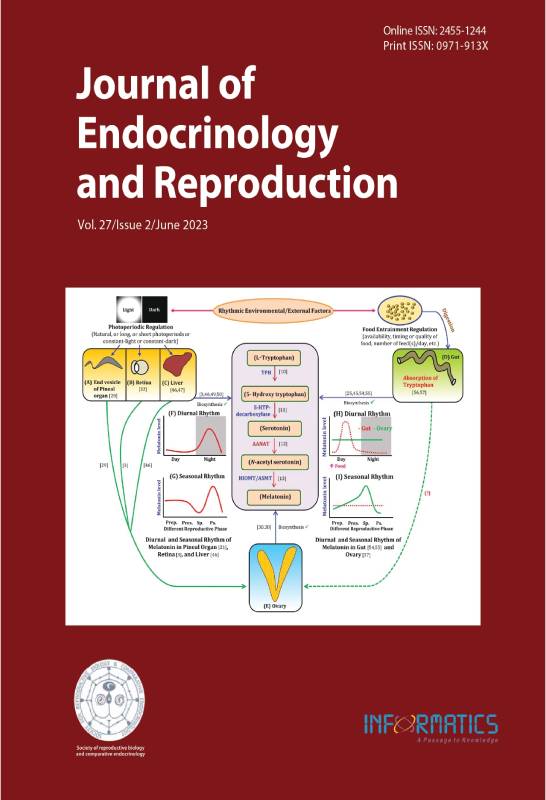
Pinealocytes of the pineal gland in vertebrates mainly synthesize melatonin (5-methoxy-N-acetyl-tryptamine). Moreover, melatonin is synthesized in several extra-pineal cells, including the photoreceptor cells of the retina, the cells of the gut, and the hepatocytes of the liver in different vertebrates, including fish species. One of the remarkable features of pineal and retinal melatonin is that it is produced rhythmically in synchronization with the environmental Light-Dark (LD) cycle, with a daily nighttime peak. However, the melatonin synthesis in tissue/cells from the extra-pineal and extra-retinal origin(s) may not always undergo photoperiod-regulated daily variations but is also dependent on the environmental food entrainment factors (in the gut), acting as the most reliable synchronizer(s) in its daily rhythm features. Moreover, the regulation of the liver and ovary (important for fish reproduction) is unclear. In this review, we attempt a comparative account of the nature and regulation of endogenous melatonin synthesis between a source like the pineal gland and many other nonpineal origins, which have gained serious attention in the last ten years. We also review the functions of melatonin in regulating fish ovarian growth and maturation. The physiological melatonin levels, manipulated either endogenously (by photoperiodic modulations) or exogenously (by injections or by feeds), have tremendous effects on reproductive events in fish at the age of its first maturity, as revealed in recent findings. Characterization and identification of the importance of pineal gland melatonin in the growth of the oocytes via the hypothalamic-pituitary-gonadal axis have been explored several years back. The identification of melatonin receptors about fourteen years back on the wall of developing oocyte spurt the breakthrough, which introduced the concept of direct control of melatonin on developing oocytes. Thus, this review gains uniqueness by addressing the latest developments recorded in the field of melatonin and fish reproduction, particularly in improving oocyte maturation. Nonetheless, an attempt has been made to underline approaches that need to be developed to apply the molecule in large-scale aquaculture.
Downloads
Metrics
Downloads
Published
How to Cite
Issue
Section
References
Klein DC. Arylalkylamine N-acetyltransferase: “The Timezyme.” J Biol Chem. 2007; 282(7):4233–7. https:// doi.org/10.1074/jbc.R600036200 PMid:17164235 DOI: https://doi.org/10.1074/jbc.R600036200
Falcón J, Besseau L, Magnanou E, et al. Melatonin, the timekeeper: Biosynthesis and effects in fish. Cybium. 2011; 35(1):3–18. https://doi.org/10.26028/cybium/ 2011-351-001
Maitra SK, Mukherjee S, Hasan KN. Melatonin: Endogenous sources and role in the regulation of fish reproduction. In: Catalá Á, editor. Indoleamines: Sources, role in biological processes and health effects. New York. Nova Science Publishers, Inc; 2015. p. 43–77.
Maitra SK, Chattoraj A, Mukherjee S, Moniruzzaman M. Melatonin: A potent candidate in the regulation of fish oocyte growth and maturation. Gen Comp Endocrinol. 2013; 181(1):215–22. https://doi.org/10.1016/j. ygcen.2012.09.015 PMid:23046602 DOI: https://doi.org/10.1016/j.ygcen.2012.09.015
Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005; 9(1):11–24. https://doi.org/10.1016/j.smrv.2004.08.001 PMid:15649735 DOI: https://doi.org/10.1016/j.smrv.2004.08.001
Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol. 2009; 44(4):175– 200. https://doi.org/10.1080/10409230903044914 PMid:19635037 DOI: https://doi.org/10.1080/10409230903044914
Tamura H, Nakamura Y, Korkmaz A, et al. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil Steril. 2009; 92(1):328-43. https:// doi.org/10.1016/j.fertnstert.2008.05.016 PMid:18804205 DOI: https://doi.org/10.1016/j.fertnstert.2008.05.016
Cipolla-Neto J, Amaral FG, Soares JM, et al. The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology. 2022; 112(2):115–29. https://doi.org/10.1159/000516148 PMid:33774638 DOI: https://doi.org/10.1159/000516148
Hazlerigg D. What is the role of melatonin within the anterior pituitary? J Endocrinol. 2001; 170(3):493–501. https://doi.org/10.1677/joe.0.1700493 PMid:11524229 DOI: https://doi.org/10.1677/joe.0.1700493
Lovenberg W, Jequier E, Sjoerdsma A. Tryptophan hydroxylation: Measurement in the pineal gland, brainstem, and carcinoid tumour. Science. 1967; 155(3759):217–9. https://doi.org/10.1126/science. 155.3759.217 PMid:6015530 DOI: https://doi.org/10.1126/science.155.3759.217
Lovenberg W, Weissbach H, Udenfriend S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962; 237(1):89–93. https://doi.org/10.1016/S0021-9258(18)81366-7 PMid:14466899 DOI: https://doi.org/10.1016/S0021-9258(18)81366-7
Voisin P, Namboodiri MA, Klein DC. Arylamine N-acetyltransferase and arylalkylamine N-acetyltransferase in the mammalian pineal gland. J Biol Chem. 1984; 259(17):10913–8. https://doi.org/10.1016/S0021-9258(18)90600-9 PMid:6469990 DOI: https://doi.org/10.1016/S0021-9258(18)90600-9
Axelrod J, Weissbach H. Enzymatic o-methylation of n-acetylserotonin to melatonin. Science. 1960; 131(3409):1312. https://doi.org/10.1126/science. 131.3409.1312 PMid:13795316 DOI: https://doi.org/10.1126/science.131.3409.1312
Lillesaar C. The serotonergic system in fish. J Chem Neuroanat. 2011; 41(4):294–308. https://doi. org/10.1016/j.jchemneu.2011.05.009 PMid:21635948 DOI: https://doi.org/10.1016/j.jchemneu.2011.05.009
Paulin C, Cazaméa‐Catalan D, Zilberman‐Peled B, et al. Subfunctionalization of arylalkylamine N‐acetyltransferases in the sea bass Dicentrarchus labrax : Two‐ones for one-two. J Pineal Res. 2015; 59(3):354–64. https://doi.org/10.1111/jpi.12266 PMid:26267754 DOI: https://doi.org/10.1111/jpi.12266
Velarde E, Cerdá-Reverter JM, Alonso-Gómez AL, et al. Melatonin-synthesizing enzymes in pineal, retina, liver, and gut of the goldfish (Carassius): mRNA expression pattern and regulation of daily rhythms by lighting conditions. Chronobiol Int. 2010; 27(6):1178201. https://doi.org/10.3109/07420528.2010.496911 PMid:20653449 DOI: https://doi.org/10.3109/07420528.2010.496911
Huang Z, Liu T, Chattoraj A, et al. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J Pineal Res. 2008; 45(4):506–14. https://doi.org/10.1111/ j.1600-079X.2008.00627.x PMid:18705647 PMCid:PMC2669754 DOI: https://doi.org/10.1111/j.1600-079X.2008.00627.x
Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003; 299(5603):76. https://doi.org/10.1126/science.1078197 PMid:12511643 DOI: https://doi.org/10.1126/science.1078197
Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004; 55(4):428–33. https://doi. org/10.1016/j.biopsych.2003.09.002 PMid:14960297 DOI: https://doi.org/10.1016/j.biopsych.2003.09.002
Bellipanni G, Rink E, Bally-Cuif L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Gene Expr Patterns. 2002; 2(3):251–6. https://doi.org/10.1016/S1567- 133X(02)00056-X PMid:12617810 DOI: https://doi.org/10.1016/S1567-133X(02)00056-X
Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M. Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol. 2010; 165(3):469–82. https:// doi.org/10.1016/j.ygcen.2009.04.026 PMid:19409900 DOI: https://doi.org/10.1016/j.ygcen.2009.04.026
Lima-Cabello E, Díaz-Casado ME, Guerrero JA, et al. A review of the melatonin functions in zebrafish physiology. J Pineal Res. 2014; 57(1):1–9. https://doi. org/10.1111/jpi.12149 PMid:24920150 DOI: https://doi.org/10.1111/jpi.12149
Hoegg S, Brinkmann H, Taylor JS, Meyer A. Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J Mol Evol. 2004; 59(2):190–203. https://doi.org/10.1007/s00239-004-2613-z PMid:15486693 DOI: https://doi.org/10.1007/s00239-004-2613-z
Volff JN. Genome evolution and biodiversity in teleost fish. Heredity. 2005; 94(3):280–94. https://doi. org/10.1038/sj.hdy.6800635 PMid:15674378 DOI: https://doi.org/10.1038/sj.hdy.6800635
Sanjita Devi H, Rajiv C, Mondal G, et al. Melatonin biosynthesizing enzyme genes (Tph1, Aanat1, Aanat2, and Hiomt) and their temporal pattern of expression in the brain and gut of tropical carp in natural environmental conditions. Cogent Biol. 2016; 2(1):1-18. https://doi.org/ 10.1080/23312025.2016.1230337 DOI: https://doi.org/10.1080/23312025.2016.1230337
Pomianowski K, Gozdowska M, Burzyński A, et al. A study of aanat and asmt expression in the threespined stickleback eye and skin: Not only “on the way to melatonin.” Comp Biochem Physiol Part A. 2020; 241:110635. https://doi.org/10.1016/j.cbpa.2019.110635 PMid:31841711 DOI: https://doi.org/10.1016/j.cbpa.2019.110635
Maitra SK, Pal PK. Melatonin rhythms in the pineal and non-pineal tissues and their physiological implications in subtropical fish. Biol Rhythm Res. 2017; 48(5):757– 76. https://doi.org/10.1080/09291016.2017.1345453 DOI: https://doi.org/10.1080/09291016.2017.1345453
Bhattacharya S, Dey R, Basu A, et al. The structure of the pineal complex in a common Indian teleost, Catla catla : Evidence for pineal‐induced inhibition of testicular function within an annual reproductive cycle. Endocr Res. 2003; 29(2):141–56. https://doi.org/10.1081/ERC-120022295 PMid:12856801 DOI: https://doi.org/10.1081/ERC-120022295
Maitra SK, Chattoraj A, Bhattacharyya S. Implication of melatonin in oocyte maturation in Indian major carp Catla catla. Fish Physiol Biochem. 2005; 31(2– 3):201–7. https://doi.org/10.1007/s10695-006-0025-2 PMid:20035459 DOI: https://doi.org/10.1007/s10695-006-0025-2
Rajiv C, Sanjita Devi H, Mondal G, et al. Cloning, phylogenetic analysis and tissue distribution of melatonin bio-synthesizing enzyme genes ( Tph1, Aanat1, Aanat2 and Hiomt) in a tropical carp, Catla catla. Biol Rhythm Res. 2016; 48(3):371–86. https://doi.org/10.1080/233120 25.2016.1230337 DOI: https://doi.org/10.1080/09291016.2016.1263019
Rajiv C, Sanjita Devi H, Mondal G, et al. Daily and seasonal expression profile of serum melatonin and its biosynthesizing enzyme genes (tph1, aanat1, aanat2, and hiomt) in pineal organ and retina: A study under natural environmental conditions in a tropical carp, Catla catla. J Exp Zool Part A Ecol Genet Physiol. 2017; 325(10):688– 700. https://doi.org/10.1002/jez.2061 PMid:28198154 DOI: https://doi.org/10.1002/jez.2061
Vivien-Roels B, Pévet P, Dubois MP, et al. Immunohistochemical evidence for the presence of melatonin in the pineal gland, the retina and the harderian gland. Cell Tissue Res. 1981; 217:105–15. https://doi. org/10.1007/BF00233830 PMid:7018690 DOI: https://doi.org/10.1007/BF00233830
Bubenik GA, Brown GM, Grota LJ. Immunohistochemical localization of melatonin in the rat Harderian gland. J Histochem Cytochem. 1976; 24(11):1173–7. https://doi.org/10.1177/24.11.63506 PMid:63506 DOI: https://doi.org/10.1177/24.11.63506
Raĭkhlin NT, Kvetnoĭ IM. Bleaching effects of extracts of mucosal and submucosal layers of the human appendix on the melanophores of frog skin. Biull EKsp Biol Med. 1974; 78(8):114–7. PMid: 4433746
Mauriz JL, Molpeceres V, García-Mediavilla MV et al. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. J Pineal Res. 2007; 42(3):222–30. https://doi. org/10.1111/j.1600-079X.2006.00409.x PMid:17349019 DOI: https://doi.org/10.1111/j.1600-079X.2006.00409.x
Sanjita Devi H, Rajiv C, Mondal G, et al. Influence of photoperiod variations on the mRNA expression pattern of melatonin bio-synthesizing enzyme genes in the pineal organ and retina: A study about the serum melatonin profile in the tropical carp Catla catla. J Fish Biol. 2022; 101(6):1569–81. https://doi.org/10.1111/jfb.15234 PMid:36205436 DOI: https://doi.org/10.1111/jfb.15234
Hasan KN, Moniruzzaman M, Maitra SK. Melatonin concentrations in relation to oxidative status and oocyte dynamics in the ovary during different reproductive phases of an annual cycle in carp Catla catla. Theriogenology. 2014; 82(8):1173–85. https://doi.org/10.1016/j.theriogenology.2014.08.001 PMid:25201351 DOI: https://doi.org/10.1016/j.theriogenology.2014.08.001
Khan ZA, Yumnamcha T, Rajiv C, et al. Melatonin biosynthesizing enzyme genes and clock genes in the ovary and whole brain of zebrafish (Danio rerio): Differential expression and a possible interplay. Gen Comp Endocrinol. 2016; 233:16–31. https://doi.org/10.1016/j. ygcen.2016.05.014 PMid:27179881 DOI: https://doi.org/10.1016/j.ygcen.2016.05.014
Mukherjee S, Maitra SK. Gut melatonin in vertebrates: Chronobiology and physiology. Front Endocrinol. 2015; 6:112. https://doi.org/10.3389/fendo.2015.00112 PMid:26257705 PMCid:PMC4510419 DOI: https://doi.org/10.3389/fendo.2015.00112
Fernández-Durán B, Ruibal C, Polakof S, et al. Evidence for arylalkylamine N-acetyltransferase (AANAT2) expression in rainbow trout peripheral tissues with emphasis in the gastrointestinal tract. Gen Comp Endocrinol. 2007; 152(2–3):289–94. https://doi. org/10.1016/j.ygcen.2006.12.008 PMid:17292900 DOI: https://doi.org/10.1016/j.ygcen.2006.12.008
Stefulj J, Hörtner M, Ghosh M, et al. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J Pineal Res. 2001; 30(4):243–7. https://doi.org/10.1034/j.1600-079X.2001.300408.x PMid:11339514 DOI: https://doi.org/10.1034/j.1600-079X.2001.300408.x
Nisembaum LG, Tinoco AB, Moure AL, et al. The arylalkylamine- N-acetyltransferase (AANAT) acetylates dopamine in the digestive tract of goldfish: A role in intestinal motility. Neurochem Int. 2013; 62(6):873– 80. https://doi.org/10.1016/j.neuint.2013.02.023 PMid:23466408 DOI: https://doi.org/10.1016/j.neuint.2013.02.023
Muñoz-Pérez JL, López-Patiño MA, Álvarez-Otero R, et al. Characterization of melatonin synthesis in the gastrointestinal tract of rainbow trout (Oncorhynchus mykiss): Distribution, relation with serotonin, daily rhythms and photoperiod regulation. J Comp Physiol B. 2016; 186(4):471–84. https://doi.org/10.1007/s00360-016-0966-4 PMid:26873742 DOI: https://doi.org/10.1007/s00360-016-0966-4
Mondal G, Dharmajyoti Devi S, Khan ZA, et al. The influence of feeding on the daily rhythm of mRNA expression on melatonin bio-synthesizing enzyme genes and clock-associated genes in the zebrafish (Danio rerio) gut. Biol Rhythm Res. 2022; 53(7):1073–90. https://doi.org/10.1080/09291016.2021.1905989 DOI: https://doi.org/10.1080/09291016.2021.1905989
Mukherjee S, Maitra SK. Effects of starvation, re-feeding and timing of food supply on daily rhythm features of gut melatonin in carp (Catla catla). Chronobiol Int. 2015; 32(9):1264–77. https://doi.org/10.3109/07420528. 2015.1087020 PMid:26513010 DOI: https://doi.org/10.3109/07420528.2015.1087020
Hasan KN, Pal PK, Maitra SK. Diurnal and seasonal profiles of melatonin in the liver and their correlates of ovarian functions under natural photo-thermal conditions in adult carp Catla catla. Biol Rhythm Res. 2016; 47(6):947–65. https://doi.org/10.1080/09291016.2016.1 212535 DOI: https://doi.org/10.1080/09291016.2016.1212535
Hasan KN, Pal PK, Maitra SK. Temporal relationship between the levels of melatonin and different antioxidants in the liver of a surface feeding carp Catla catla. Biol Rhythm Res. 2020; 51(3):373–91. https://doi. org/10.1080/09291016. 2018.1533728 DOI: https://doi.org/10.1080/09291016.2018.1533728
Falcón J, Thibault C, Martin C, et al. Regulation of melatonin production by catecholamines and adenosine in a photoreceptive pineal organ. An in vitro study in the pike and the trout. J Pineal Res. 1991; 11(3–4):123–34. https://doi.org/10.1111/j.1600-079X.1991.tb00467.x PMid:1795221 DOI: https://doi.org/10.1111/j.1600-079X.1991.tb00467.x
Seth M, Maitra SK. Importance of light in the temporal organization of photoreceptor proteins and melatonin-producing system in the pineal of carp Catla catla. Chronobiol Int. 2010; 27(3):463–86. https://doi. org/10.3109/07420521003666416 PMid:20524796 DOI: https://doi.org/10.3109/07420521003666416
Falcón J, Collin JP. Pineal-retinal relationships: rhythmic biosynthesis and immunocytochemical localization of melatonin in the retina of the pike (Esox lucius). Cell Tissue Res. 1991; 265:601–9. https://doi.org/10.1007/ BF00340884 DOI: https://doi.org/10.1007/BF00340884
Dubocovich ML. Characterization of a retinal melatonin receptor. J Pharmacol Exp Ther. 1985; 234(2):395–401. PMid: 2991499
Wiechmann AF. Melatonin: Parallels in the pineal gland and retina. Exp Eye Res. 1986; 42(6):507–27. https://doi. org/10.1016/0014-4835(86)90042-4 PMid:3013666 DOI: https://doi.org/10.1016/0014-4835(86)90042-4
Kulczykowska E, Kalamarz H, Warne JM, Balment RJ. Day-night specific binding of 2-[125I]Iodomelatonin and melatonin content in gill, small intestine and kidney of three fish species. J Comp Physiol B. 2006; 176(4):277–85. https://doi.org/10.1007/s00360-005- 0049-4 PMid:16307275 DOI: https://doi.org/10.1007/s00360-005-0049-4
Mukherjee S, Moniruzzaman M, Maitra SK. Daily and seasonal profiles of gut melatonin and their temporal relationship with pineal and serum melatonin in carp Catla catla under natural photo-thermal conditions. Biol Rhythm Res. 2014; 45(2):301–15. https://doi.org/10.108 0/09291016.2013.817139 DOI: https://doi.org/10.1080/09291016.2013.817139
Mukherjee S, Moniruzzaman M, Maitra SK. Impact of artificial lighting conditions on the diurnal profiles of gut melatonin in a surface-dwelling carp (Catla catla). Biol Rhythm Res. 2014; 45(6):831–48. https://doi.org/10 .1080/09291016.2014.923618 DOI: https://doi.org/10.1080/09291016.2014.923618
Mukherjee S, Maitra SK. Daily profiles of serum and gastrointestinal melatonin in response to daytime or night-time supply of tryptophan-rich diet in carp (Catla catla). Biol Rhythm Res. 2018; 49(2):315–27. https://doi. org/10.1080/09291016.2017.1361157 DOI: https://doi.org/10.1080/09291016.2017.1361157
Lepage O, Larson ET, Mayer I, Winberg S. Tryptophan affects both gastrointestinal melatonin production and interrenal activity in stressed and nonstressed rainbow trout. J Pineal Res. 2005; 38(4):264–71. https://doi. org/10.1111/j.1600-079X.2004.00201.x PMid:15813903 DOI: https://doi.org/10.1111/j.1600-079X.2004.00201.x
Yasmin F, Sutradhar S, Das P, Mukherjee S. Gut melatonin: A potent candidate in the diversified journey of melatonin research. Gen Comp Endocrinol. 2021; 303:113693. https://doi.org/10.1016/j. ygcen.2020.113693PMid:33309697 DOI: https://doi.org/10.1016/j.ygcen.2020.113693
Lake JS. Rearing experiments with five species of Australian freshwater fishes. I. Inducement to spawning. Aust J Mar Freshw Res. 1967; 18(2):137–54. https://doi. org/10.1071/MF9670137 DOI: https://doi.org/10.1071/MF9670137
Schwassmann HO. Times of annual spawning and reproductive strategies in Amazonian fishes. In: Thorpe JE, editor. Rhythmic Activities of Fishes. London. Academic Press; 1978 pp. 187–200.
Lam TJ. Environmental influences on gonadal activity in fish. Fish Physiol. 1983; 9:65-116. https://doi. org/10.1016/S1546-5098(08)60302-7 DOI: https://doi.org/10.1016/S1546-5098(08)60302-7
Hyder M. Gonadal and reproductive patterns in Tilapia leucosticta (Teleostei: Cichlidae) in an equatorial lake, Lake Naivasha (Kenya). J Zool. 1970; 162(2):179–95. https://doi.org/10.1111/j.1469-7998.1970.tb01263.x DOI: https://doi.org/10.1111/j.1469-7998.1970.tb01263.x
Bayarri MJ, Garcia-Allegue R, Muñoz-Cueto J, et al. Melatonin binding sites in the brain of European sea bass (Dicentrarchus labrax). Zoolog Sci. 2004; 21(4):427–34. https://doi.org/10.2108/zsj.21.427 PMid:15118230 DOI: https://doi.org/10.2108/zsj.21.427
Vasal S, Sundararaj BI. Response of the ovary in the catfish, Heteropneustes fossilis (Bloch), to various combinations of photoperiod and temperature. J Exp Zool. 1976; 197(2):247–63. https://doi.org/10.1002/ jez.1401970206 PMid:965909 DOI: https://doi.org/10.1002/jez.1401970206
Moav R, Wohlfarth G. Carp breeding in Israel. In: Moav R, editor. Agricultural Genetics. New York. John Wiley and Sons; 1973. p. 295–318.
Billard R, Breton B. Rhythms of reproduction in teleost fish. In: Thorpe JE, editor. Rhythmic activities of fishes. London. Academic Press; 1978. p. 31–53.
Bhattacharya S, Chattoraj A, Maitra SK. Melatonin in the regulation of annual testicular events in carp Catla catla : Evidence from the studies on the effects of exogenous melatonin, continuous light, and continuous darkness. Chronobiol Int. 2007; 24(4):629–50. https:// doi.org/10.1080/07420520701534665 PMid:17701677 DOI: https://doi.org/10.1080/07420520701534665
Dey R, Bhattacharya S, Maitra SK. Temporal pattern of ovarian activity in a major carp Catla catla and its possible environmental correlate in an annual cycle. Biol Rhythm Res. 2004; 35(4–5):329–53. https://doi. org/10.1080/09291010400003792 DOI: https://doi.org/10.1080/09291010400003792
Bhattacharya S. Recent advances in the hormonal regulation of gonadal maturation and spawning in fish. Curr Sci. 1999; 76(3):342-9.
Pang Y, Ge W. Gonadotropin and activin enhance maturational competence of oocytes in the zebrafish (Danio rerio). Biol Reprod. 2002; 66(2):259–65. https://doi. org/10.1095/biolreprod66.2.259 PMid:11804937 DOI: https://doi.org/10.1095/biolreprod66.2.259
Nagahama Y. 17α,20β-Dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in fish oocytes: Mechanisms of synthesis and action. Steroids. 1997; 62:190–6. https://doi.org/10.1016/S0039- 128X(96)00180-8 PMid:9029736 DOI: https://doi.org/10.1016/S0039-128X(96)00180-8
Yamashita M, Fukada S, Yoshikuni M, et al. Purification and characterization of maturation-promoting factor in fish. Dev Biol. 1992; 149(1):8–15. https://doi. org/10.1016/0012-1606(92)90259-J PMid:1728595 DOI: https://doi.org/10.1016/0012-1606(92)90259-J
Tokumoto T, Tokumoto M, Horiguchi R, et al. Diethylstilbestrol induces fish oocyte maturation. Proc Natl Acad Sci USA. 2004; 101(10):3686–90. https://doi.org/10.1073/pnas.0400072101 PMid:14990787 PMCid:PMC373523 DOI: https://doi.org/10.1073/pnas.0400072101
Bhattacharyya S, Dey R, Maitra SK. Photoperiodic regulation of annual testicular events in the Indian major carp Catla catla. Acta Zool. 2005; 86(2):71–9. https:// doi.org/10.1111/j.1463-6395.2005.00188.x DOI: https://doi.org/10.1111/j.1463-6395.2005.00188.x
Dey R, Bhattacharya S, Maitra SK. Importance of photoperiods in the regulation of ovarian activities in Indian major carp Catla catla in an annual cycle. J Biol Rhythms. 2005; 20(2):145–58. https://doi. org/10.1177/0748730404272925 PMid:15834111 DOI: https://doi.org/10.1177/0748730404272925
Mayer I, Bornestaf C, Borg B. Melatonin in non-mammalian vertebrates: Physiological role in reproduction? Comp Biochem Physiol. 1997; 118A(3):515–31. https:// doi.org/10.1016/S0300-9629(96)00468-9 DOI: https://doi.org/10.1016/S0300-9629(96)00468-9
Gaildrat P, Becq F, Falcón J. First cloning and functional characterization of a melatonin receptor in fish brain: A novel one? J Pineal Res. 2002; 32(2):74–84. https://doi. org/10.1034/j.1600-079x.2002.1817.x PMid:12071471 DOI: https://doi.org/10.1034/j.1600-079x.2002.1817.x
Gaildrat P, Falcón J. Melatonin receptors in the pituitary of a teleost fish: mRNA expression, 2-[125I]iodomelatonin binding and cyclic AMP response. Neuroendocrinology. 2000; 72(1):57–66. https://doi.org/10.1159/000054571 PMid:10940739 DOI: https://doi.org/10.1159/000054571
Amano M, Iigo M, Ikuta K, et al. Roles of melatonin in gonadal maturation of under yearling precocious male masu salmon. Gen Comp Endocrinol. 2000; 120(2):190–7. https://doi.org/10.1006/gcen.2000.7547 PMid:11078630 DOI: https://doi.org/10.1006/gcen.2000.7547
Chattoraj A, Seth M, Basu A, et al. Temporal relationship between the circulating profiles of melatonin and ovarian steroids under natural photo-thermal conditions in an annual reproductive cycle in carp Catla catla. Biol Rhythm Res. 2009; 40(4):347–59. https://doi. org/10.1080/09291010802404218 DOI: https://doi.org/10.1080/09291010802404218
Iigo M, Kobayashi M, Ohtani-Kaneko R, et al. Characteristics, day-night changes, subcellular distribution and localization of melatonin binding sites in the goldfish brain. Brain Res. 1994; 644(2):213–20. https:// doi.org/10.1016/0006-8993(94)91682-9 PMid:8050032 DOI: https://doi.org/10.1016/0006-8993(94)91682-9
Yumnamcha T, Khan ZA, Rajiv C, et al. Interaction of melatonin and gonadotropin-inhibitory hormone on the zebrafish brain-pituitary-reproductive axis. Mol Reprod Dev. 2017; 84(5):389–400. https://doi.org/10.1002/ mrd.22795 PMid:28295807 DOI: https://doi.org/10.1002/mrd.22795
Chattoraj A, Bhattacharyya S, Basu D, et al. Melatonin accelerates Maturation Inducing Hormone (MIH): Induced oocyte maturation in carps. Gen Comp Endocrinol. 2005; 140(3):145–55. https://doi. org/10.1016/j.ygcen.2004.10.013 PMid:15639142 DOI: https://doi.org/10.1016/j.ygcen.2004.10.013
Mondal P, Hasan KN, Pal PK, Maitra SK. Influences of exogenous melatonin on the oocyte growth and oxidative status of the ovary during different reproductive phases of an annual cycle in carp Catla catla. Theriogenology. 2017; 87:349–59. https://doi.org/10.1016/j.theriogenology. 2016.09.021 PMid:27743691 DOI: https://doi.org/10.1016/j.theriogenology.2016.09.021
Panchal R, Rani S. Effect of melatonin and pinealectomy on gonadal activity during the pre-spawning period of catfish (Heteropneustes fossilis). J Environ Biol. 2019; 40(4):698–704. https://doi.org/10.22438/jeb/40/4/ MRN-925 DOI: https://doi.org/10.22438/jeb/40/4/MRN-925
Kim JH, Park JW, Kwon JY. Effects of exogenous melatonin on the reproductive activities of Nile tilapia, Oreochromis niloticus. Biol Rhythm Res. 2018; 49(3):392–404. https://doi.org/10.1080/09291016.2017. 1366715 DOI: https://doi.org/10.1080/09291016.2017.1366715
Badruzzaman M, Ikegami T, Amin AKMR, Shahjahan M. Melatonin inhibits reproductive activity through changes of serotonergic activity in the brain of freshwater catfish (Mystus cavasius). Aquaculture. 2020; 526:735378. https://doi.org/10.1016/j.aquaculture.2020.735378 DOI: https://doi.org/10.1016/j.aquaculture.2020.735378
Falcón J, Besseau L, Sauzet S, Boeuf G. Melatonin effects on the hypothalamo–pituitary axis in fish. Trends Endocrinol Metab. 2007; 18(2):81–8. https://doi. org/10.1016/j.tem.2007.01.002 PMid:17267239 DOI: https://doi.org/10.1016/j.tem.2007.01.002
Mazurais D, Porter M, Lethimonier C, et al. Effects of melatonin on liver estrogen receptor and vitellogenin expression in rainbow trout: An in vitro and in vivo study. Gen Comp Endocrinol. 2000; 118(2):344–53. https://doi.org/10.1006/gcen.2000.7472 PMid:10890573 DOI: https://doi.org/10.1006/gcen.2000.7472
Sherwood N, Eiden L, Brownstein M, et al. Characterization of a teleost gonadotropin-releasing hormone. Proc Natl Acad Sci USA. 1983; 80(9):2794–8. https://doi.org/10.1073/pnas.80.9.2794 PMid:6341999 PMCid:PMC393915 DOI: https://doi.org/10.1073/pnas.80.9.2794
Reiter RJ, Tan DX, Korkmaz A. The circadian melatonin rhythm and its modulation: Possible impact on hypertension. J Hypertens. 2009; 27(Suppl 6):S17-S20. https://doi.org/10.1097/01.hjh.0000358832.41181.bf PMid:19633446 DOI: https://doi.org/10.1097/01.hjh.0000358832.41181.bf
Malpaux B, Thiéry JC, Chemineau P. Melatonin and the seasonal control of reproduction. Reprod Nutr Dev 1999; 39:355–66. https://doi.org/10.1051/rnd:19990308 PMid:10420438 DOI: https://doi.org/10.1051/rnd:19990308
Revel FG, Ansel L, Klosen P, et al. Kisspeptin: A key link to seasonal breeding. Rev Endocr Metab Disord. 2007; 8:57–65. https://doi.org/10.1007/s11154-007-9031-7 PMid:17380397 DOI: https://doi.org/10.1007/s11154-007-9031-7
Roy D, Belsham DD. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1–7 GnRH neurons. J Biol Chem. 2002; 277(1):251–8. https:// doi.org/10.1074/jbc.M108890200 PMid:11684691 DOI: https://doi.org/10.1074/jbc.M108890200
Tsutsui K, Bentley GE, Ubuka T, et al. The general and comparative biology of Gonadotropin-Inhibitory Hormone (GnIH). Gen Comp Endocrinol. 2007; 153(1– 3):365–70. https://doi.org/10.1016/j.ygcen.2006.10.005 PMid:17141777 DOI: https://doi.org/10.1016/j.ygcen.2006.10.005
Ubuka T, Son YL, Tobari Y, Tsutsui K. Gonadotropininhibitory hormone action in the brain and pituitary. Front Endocrinol. 2012; 3:1–14. https://doi.org/10.3389/ fendo.2012.00148 PMid:23233850 PMCid:PMC3515997
Kim JH, Park JW, Jin YH, et al. Effect of melatonin on GnIH precursor gene expression in Nile tilapia, Oreochromis niloticus. Biol Rhythm Res. 2018; 49(2):303–13. https://doi.org/10.1080/09291016.2017.1 357336 DOI: https://doi.org/10.1080/09291016.2017.1357336
Imamura S, Hur SP, Takeuchi Y, et al. Effect of short- and long-term melatonin treatments on the reproductive activity of the tropical damselfish Chrysiptera cyanea. Fish Physiol Biochem. 2022; 48:253–62. https://doi. org/10.1007/s10695-022-01051-x PMid:35099686 DOI: https://doi.org/10.1007/s10695-022-01051-x
Tsutsui K, Bentley GE, Bedecarrats G, et al. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol. 2010; 31(3):284–95. https://doi. org/10.1016/j.yfrne.2010.03.001 PMid:20211640 DOI: https://doi.org/10.1016/j.yfrne.2010.03.001
Khan IA, Thomas P. Melatonin influences gonadotropin ii secretion in the Atlantic croaker (Micropogonias undulatus). Gen Comp Endocrinol. 1996; 104(2):231–42. https://doi.org/10.1006/gcen.1996.0166 PMid:8930614 DOI: https://doi.org/10.1006/gcen.1996.0166
Popek W, Łuszczek–Trojnar E, Drąg-Kozak E, et al. Effect of the pineal gland and melatonin on dopamine release from perifused hypothalamus of mature female carp during spawning and winter regression. Acta Ichthyol Piscat. 2005; 35(2):65–71. https://doi. org/10.3750/AIP2005.35.2.01 DOI: https://doi.org/10.3750/AIP2005.35.2.01
Sébert ME, Legros C, Weltzien FA, et al. Melatonin activates brain dopaminergic systems in the eel with an inhibitory impact on reproductive function. J Neuroendocrinol. 2008; 20(7):917–29. https://doi. org/10.1111/j.1365-2826.2008.01744.x PMid:18445127 DOI: https://doi.org/10.1111/j.1365-2826.2008.01744.x
Amano M, Iigo M, Ikuta K, et al. Disturbance of plasma melatonin profile by high dose melatonin administration inhibits testicular maturation of precocious male masu salmon. Zoolog Sci. 2004; 21(1):79–85. https://doi.org/10.2108/0289-0003(2004)21[79:DOPMPB]2.0.CO;2 PMid:14745107 DOI: https://doi.org/10.2108/0289-0003(2004)21[79:DOPMPB]2.0.CO;2
Gaildrat P, Falcón J. Expression of melatonin receptors and 2-[125I]Iodomelatonin binding sites in the pituitary of a teleost fish. In: Olcese J, editor. Melatonin After Four Decades. New York. Kluwer Academic/Plenum Publishers; 1999 pp. 61–72. https://doi.org/10.1007/0- 306-46814-X_8 PMid:10810501 DOI: https://doi.org/10.1007/0-306-46814-X_8
Takahashi T, Ogiwara K. Roles of melatonin in the teleost ovary: A review of the current status. Comp Biochem Physiol Part A. 2021; 254:110907. https://doi. org/10.1016/j.cbpa.2021.110907 PMid:33482340 DOI: https://doi.org/10.1016/j.cbpa.2021.110907
Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008; 50(SUPPL.1):S195–219. https://doi.org/10.1111/j.1440-169X.2008.01019.x PMid:18482399 DOI: https://doi.org/10.1111/j.1440-169X.2008.01019.x
Mukherjee D, Bhattacharya S. Effects of gonadotropin hormone on fish ovarian 17β-hydroxysteroid dehydrogenase activity. In Proceedings of the 1st International symposium of life sciences; 1983.
Hong LY, Hong WS, Zhu WB, et al. Cloning and expression of melatonin receptors in the mudskipper Boleophthalmus pectinirostris: Their role in synchronizing its semilunar spawning rhythm. Gen Comp Endocrinol. 2014; 195:138–50. https://doi. org/10.1016/j.ygcen.2013.11.004 PMid:24239555 DOI: https://doi.org/10.1016/j.ygcen.2013.11.004
Patiño R, Yoshizaki G, Thomas P, Kagawa H. Gonadotropic control of ovarian follicle maturation: The two-stage concept and its mechanisms. Comp Biochem Physiol Part B. 2001; 129(2–3):427–39. https://doi. org/10.1016/S1096-4959(01)00344-X PMid:11399477 DOI: https://doi.org/10.1016/S1096-4959(01)00344-X
Thomas P, Pang Y, Zhu Y, et al. Multiple rapid progestin actions and progestin membrane receptor subtypes in fish. Steroids. 2004; 69(8):567–73. https://doi. org/10.1016/j.steroids.2004.05.004 PMid:15288771 DOI: https://doi.org/10.1016/j.steroids.2004.05.004
Zhu Y, Rice CD, Pang Y, et al. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci. 2003; 100(5):2231– 6. https://doi.org/10.1073/pnas.0336132100 PMid: 12574519 PMCid:PMC151323 DOI: https://doi.org/10.1073/pnas.0336132100
Sánchez-Barceló EJ, Cos S, Mediavilla D, et al. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005; 38(4):217–22. https://doi.org/10.1111/ j.1600-079X.2004.00207.x PMid:15813897 DOI: https://doi.org/10.1111/j.1600-079X.2004.00207.x
Wu CS, Leu SF, Yang HY, Huang BM. Melatonin inhibits the expression of steroidogenic acute regulatory protein and steroidogenesis in MA-10 cells. J Androl. 2001; 22(2):245–54. https://doi.org/https://doi. org/10.1002/j.1939-4640.2001.tb02177.x
Chattoraj A, Seth M, Maitra SK. Localization and dynamics of Mel1a melatonin receptor in the ovary of carp Catla catla in relation to serum melatonin levels. Comp Biochem Physiol Part A. 2009; 152(3):327–33. https://doi.org/10.1016/j.cbpa.2008.11.010 PMid:19068233 DOI: https://doi.org/10.1016/j.cbpa.2008.11.010
Carnevali O, Gioacchini G, Maradonna F, et al. Melatonin induces follicle maturation in Danio rerio. PLoS One. 2011; 6(5):e19978. https://doi.org/10.1371/journal. pone.0019978 PMid:21647435 PMCid:PMC3102064 DOI: https://doi.org/10.1371/journal.pone.0019978
Moniruzzaman M, Hasan KN, Maitra SK. Melatonin actions on ovaprim (synthetic GnRH and domperidone)- induced oocyte maturation in carp. Reproduction. 2016; 151(4):285–96. https://doi.org/10.1530/REP-15-0391 PMid:26729919 DOI: https://doi.org/10.1530/REP-15-0391
Woo MM, Tai CJ, Kang SK, et al. Direct action of melatonin in human granulosa-luteal cells. J Clin Endocrinol Metab. 2001; 86(10):4789–97. https://doi.org/10.1210/jcem.86.10.7912 PMid:11600542 DOI: https://doi.org/10.1210/jcem.86.10.7912
Soares JM, Masana MI, Erşahin Ç, Dubocovich ML. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J Pharmacol Exp Ther. 2003; 306(2):694–702. https://doi.org/10.1124/ jpet.103.049916 PMid:12721330 DOI: https://doi.org/10.1124/jpet.103.049916
Moniruzzaman M, Maitra SK. Influence of altered photoperiods on serum melatonin and its receptors (MT1 and MT2) in the brain, retina, and ovary in carp Catla catla. Chronobiol Int. 2012; 29(2):175–88. https://doi. org/10.3109/07420528.2011.645753 PMid:22324556 DOI: https://doi.org/10.3109/07420528.2011.645753
Chai K, Liu X, Zhang Y, Lin H. Day-night and reproductive cycle profiles of melatonin receptor, kiss, and gnrh expression in orange-spotted grouper (Epinephelus coioides). Mol Reprod Dev. 2013; 80(7):535–48. https:// doi.org/10.1002/mrd.22191 PMid:23661545 DOI: https://doi.org/10.1002/mrd.22191
Jin YH, Park JW, Kim JH, Kwon JY. The expression pattern of melatonin receptor 1a gene during early life stages in the Nile tilapia (Oreochromis niloticus). Dev Reprod. 2013; 17(1):45–53. https://doi. org/10.12717/DR.2013.17.1.045 PMid:25949120 PMCid:PMC4282221 DOI: https://doi.org/10.12717/DR.2013.17.1.045
Ogiwara K, Takahashi T. A dual role for melatonin in medaka ovulation: Ensuring prostaglandin synthesis and actin cytoskeleton rearrangement in follicular cells. Biol Reprod. 2016; 94(3):1–15. https://doi.org/10.1095/ biolreprod.115.133827 PMid:26864196 DOI: https://doi.org/10.1095/biolreprod.115.133827
Tan DX, Chen LD, Poeggeler B, et al. Melatonin - a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993; 1:57–60.
Maitra SK, Hasan KN. The role of melatonin as a hormone and an antioxidant in the control of fish reproduction. Front Endocrinol. 2016; 7:38. https:// doi.org/10.3389/fendo.2016.00038 PMid:27199895 PMCid:PMC4854901 DOI: https://doi.org/10.3389/fendo.2016.00038
Manchester LC, Coto-Montes A, Boga JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. 2015; 59(4):403– 19. https://doi.org/10.1111/jpi.12267 PMid:26272235 DOI: https://doi.org/10.1111/jpi.12267
Bhattacharyya S, Maitra SK. Environmental correlate of the testicular events in a major carp Catla catla in an annual reproductive cycle. Biol Rhythm Res. 2006; 37(2):87–110. https://doi. org/10.1080/09291010500124605 DOI: https://doi.org/10.1080/09291010500124605
 Sona Sutradhar
Sona Sutradhar